INTRODUCTION
Although inherently a non-hepatotropic virus, a direct cytopathic effect of human immunodeficiency virus-1 (HIV) on liver, independent of retroviral drug-toxicity or viral hepatitis is known, today. HIV co-infection with hepatitis B virus (HBV), and the consequences on progression of severe liver diseases further pose a serious health issue. HIV alone, infects nearly 40 million individuals leading to about 5% cases of mortality while HBV circulates in > 400 million persons causing about one million deaths, annually worldwide[1,2]. In the co-infection cases, approximately 90% of HIV-infected individuals have serological markers of HBV and approximately 5%-40% of them are clinically chronic[3,4]. As a consequence, HBV co-infection persists in almost 25% of HIV-infected adults and approximately 50%-90% of them acquire it at birth or in early age[5].
In HIV-infected patients, the CD4-independant tissue tropism, including liver and its impact on hepatopathogenesis is well established[6]. The spectrum of HIV-induced liver diseases includes hepatitis, alcohol-associated steatohepatitis, non-alcoholic steatohepatitis, endothelialitis, necrosis and granulomatosis[7]. Moreover, HIV co-infection leads to further complications in liver diseases compared to HBV mono-infection[8]. It is found that in HIV co-infected individuals, liver-related mortality is over 17 times higher than those infected with HBV, alone[2]. Also, with the introduction of “highly active anti-retroviral therapy (HAART)” in high HBV endemic areas, greater chances of progressive liver diseases is expected in the HIV co-infected individuals[9]. Moreover, HIV co-infection severely alters the natural history of hepatitis B[9]. As a result, management of chronic hepatitis B is hampered by the late and/or improper diagnosis as well as by effectiveness of nucleos(t)ide-based anti-viral therapy, and risks of emergence of drug-resistance. While it is very rare for HBV, transmittance of drug resistant-mutants accounts for up to 15% of new HIV infections[10,11].
HIV-HEPATOTROPISM
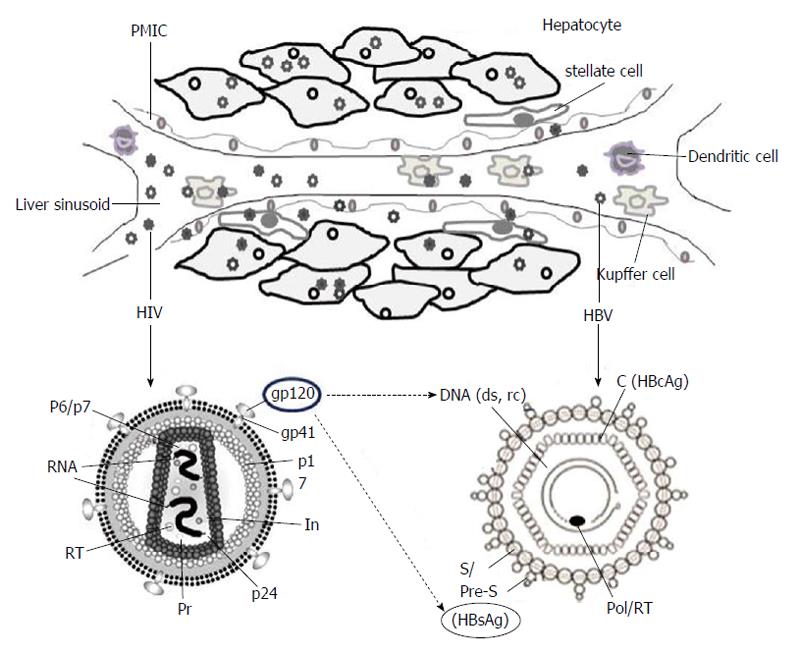
HIV infection requires interaction of the viral envelop protein (gp120) with cell surface receptor, CD4, aided by chemokine co-receptors, CCR5 and CXCR4[12,13]. While some HIV strains also require both of these co-receptors, few rare laboratory-adapted isolates are shown to utilize alternative co-receptors like, CCR1, CCR2b, CCR8, CXCR6, GPR1 and GPR15/Bob, in vitro[14]. Ample of reports has demonstrated cross-tissue tropism of HIV capable of infecting a variety of non-lymphoid tissues in vivo[15]. Further, HIV RNA, proviral-deoxyribonucleic acid (DNA) and capsid antigen (p24) have been detected in liver sinusoidal endothelial cells, Kupffer cells, portal mononuclear inflammatory cells as well as hepatocytes of infected patients (Figure 1)[16]. Very interestingly, Iser et al[17] have recently demonstrated the indispensability of CCR5 and CXCR4 in HIV entry into multiple liver cell lines. Nevertheless, the limited sensitiveness of the techniques employed as well as the sub-detectable levels of CD4 after several passages of cultured cells, cannot rule out the possibility of CD4 expressions on these cells. Therefore, in HIV-positive patients, the retroviral CD4 receptor-independent entry of hepatocytes still remains controversial. Nevertheless, another possibility of HIV to utilize an alternative liver-specific receptor is recently described[18].
Figure 1 A cartoon depiction of hepatitis B virus and human immunodeficiency virus co-infection of liver.
Intracellular locations of the two viruses in hepatocytes, liver sinusoidal endothelial cells, Kupffer cells, portal mononuclear inflammatory cells, stellate cells, dendritic cells and Kupffer cells are shown (upper panel). The structural and genomic organizations of HIV and HBV indicating the probable trans-regulation of HBV-DNA and HBsAg by HIVgp120 (lower panel). PMIC: Portal mononuclear inflammatory cell; HIV: Human immunodeficiency virus; HBV: Hepatitis B virus; DNA: Deoxyribonucleic acid; HBsAg: Hepatitis B surface antigen; HBcAg: Hepatitis B core antigen; RT: HIV reverse-transcriptase.
CO-INFECTION AND SEVERITY OF LIVER PATHOGENESIS
The spectrum of HIV-induced liver diseases includes hepatitis, alcohol-associated steatohepatitis, non-alcoholic steatohepatitis, endothelialitis, necrosis and granulomatosis[7,19]. The consequences of HIV mono-infection is also linked to liver portal obliteration with nodular regenerative hyperplasia, responsible for non-cirrhotic portal hypertension[20,21]. In adult-acquired HBV infected individuals, the secondary infection with HIV leads to the chances of developing chronic hepatitis B by six-fold compared to HBV mono-infected patients[22,23]. Furthermore, in co-infection cases, HIV severely alters the natural history of hepatitis B[3,24]. HIV co-infection significantly decreases the rate of hepatitis B e antigen (HBeAg) clearance up to five-fold and increases the level of HBV replication[9,25]. Even co-infected persons who seroconvert to protective antibody to hepatitis B surface antigen (HBsAg) [anti-hepatitis B surface antibody (HBsAb)], remain at risk of reverse-seroconversion, and subsequent reactivation of HBV[26,27]. Also, while liver cirrhosis is more common in cases of HBV and HIV co-infections, a more severe progression and complexity in the development of hepatocellular carcinoma (HCC) is also reported[28].
UNDERLYING MECHANISMS
Though the high HBV episomal DNA (cccDNA) accumulations are predictive of elevated hepatotoxicity and contrarily, the lower alanine amino transaminase (ALT) levels presenting less hepatic injury; the underlying mechanisms of HIV-induced liver pathology is largely unknown. Moreover, HBV core (C)/precore (pre-C) genetic variants, which can be more common in HIV co-infected individuals, are also suggested in disease progression[29,30]. In a closely related clinical study of HIV and hepatitis C virus co-infection cases, liver cirrhosis was found associated with higher systemic markers of gut-microbial translocation[31] that could be also true for HBV. Further, the activation of HBV-specific CD8+ cells, crucial in controlling HBV replication as well as the liver pathogenesis[32], is shown to be clearly impaired during HIV secondary infection. This might partially explain the tendency of progression of acute hepatitis B towards chronicity in HIV co-infection cases[33]. In co-infected patients with lower ALT levels compared to HBV mono-infection, the progression of cirrhosis and HCC is suggested to be related to lower CD4+ cell counts[34,35]. In addition to higher levels of HBV DNA and lower rates of spontaneous HBeAg seroconversion, severe flares of hepatitis can occur in HIV co-infected patients with low CD4+ counts who experience immune reconstitution after initiation of HAART[36]. Clinical studies in HBV and HIV co-infected patients have reported lower response rates to standard interferon (IFN)-α treatment than those with HBV mono-infection[34]. Responders tend to have a higher mean CD4+ cell count than nonresponders. It is expected that pegIFN-α will have similar or better efficacy than standard IFN-α. However, a detailed study of the mechanisms underlying adaptive immune responses in such cases is not available.
Furthermore, the CCR5 and CXCR4 co-receptors of hepatocytes as well as stellate cells are reported to involve in fibrogenic developments[36]. Very recently, the viral gp120 has been demonstrated to modulate hepatic chemokine receptors as well as stellate cell biology, and linked to liver fibrogenesis[37,38]. The gp120 binding of hepatic CXCR4 is also reported to up-regulate tumor necrosis factor-related TRAIL-R2 expression in apoptosing hepatocytes[39]. Another protein “R” of HIV is shown to modulate host transcription factors, like peroxisome proliferator-activated receptor-γ, crucial in lipid metabolism, insulin sensitivity, inflammatory processes and fibrogenesis[40].
Though several studies have demonstrated impact of HIV proteins on hepatocyte biology[20,37-40], only a few data is available on viral interactions, i.e., between HBV and HIV proteins (Figure 1). In the co-infection situation, the co-synthesized proteins of the two viruses might compete for host machinery involved in virion secretion pathways[41,42]. In ex vivo experimental set-up, the high retention of intrahepatic HBsAg had indicated its enhanced production or impaired secretion in the culture supernatant[43]. Moreover, in co-infected patients, exertion of gp120 on elevations and intracellular accumulation of HBV, DNA as well as HBsAg is proposed (Figure 1) to cause hepatotoxicity. In a clinical study, association of HBV and HIV co-infection with higher levels of HBV DNA has suggested that factors other than a direct virus-virus interaction might contribute to the increased HBV DNA levels[9]. While it has been shown that the HBV-X protein acts in alliance with HIV-tat in facilitating HIV replication[44], the synergistic effect of tat, if any, on HBV life cycle is not known.
DIAGNOSTIC AND THERAPEUTIC CHALLENGES
The primary objective of hepatitis B treatment in HIV co-infection cases is to suppress HBV viral replication and minimize progressive liver damage. Notably, in co-infected patients, HIV severely alters the natural history of hepatitis B and therefore, complicates the diagnosis and disease management. HBsAg seronegativity and anti-HBsAb seroconversion that indicate resolution of active hepatitis B, are uncommon in HIV co-infection. Further, spontaneous reverse-seroconversion of anti-HBsAb can also occur in some co-infected patients and therefore, isolated anti-HBcAb test is recommended[45]. Since co-infected patients can have high levels of HBV DNA and hepatic necroinflammation with anti-HBc but not HBsAg, it is advised to test for both seromarkers first, and if either is positive, to test for HBV DNA[46].
Although, there is a limited therapeutic options for the treatment of chronic HBV, nearly twenty five approved drugs belonging to six classes [nucleos(t)ide RT-, protease-, nonnucleos(t)ide RT-, fusion-, integrase-, and CCR5-inhibitors] are available for HIV. In the co-infected individuals, the design of therapeutic regimens, like HAART are therefore, recommended to minimize the risk of hepatotoxicity. Also, due to association of continued anti-retroviral drug therapy with liver fibrogenesis and toxicity, it is prescribed for the necessity of balancing potential toxic effects with the need for increasing the CD4+ cell counts to control the two viruses[47]. Moreover, due to the resistance of highly stable, nuclear cccDNA to the currently available nucleos(t)ide analogs (e.g., Lamivudine, Adefovir, Emtricitabine, Tenofovir, etc.), a complete elimination of HBV has not been achieved. HBV therapy is recommended for all co-infected patients with abnormal aminotransferase values or HBV DNA levels of > 2000 IU/mL. Patients with an indication for HBV treatment should be started on fully active anti-retroviral regimen that contains nucleos(t)ide analogues, regardless of the CD4 cell count, to ensure that HIV is not partially treated[48]. Unlike in HBV mono-infection cases, combinatorial treatment with pegylated-Interferon plus Adefovir (preferred over Lamivudine resistance) has not led to any success in HIV co-infected individuals[49]. Further, because Entecavir can result in emergence of drug-resistant HIV muatants, its use is restricted in co-infected patients who are on a suppressive HIV regimen[50,51]. Although, Tenofovir is the most commonly used anti-viral analog in the co-infected patients, a few studies have examined the development of resistance in HIV as well as HBV. Since, in a proportion of Tenofovir treated patients, an undetectable serum HBV DNA still circulates, Tenofovir in combination with Emtricitabine is being preferred against the two viruses[52].
Though Lamivudine, Emtricitabine and Tenofovir are efficacious against both HBV and HIV, the rate of viral cross-resistance to Lamivudine, for example, in co-infected patients is high, reaching up to 90% at 4 years[53]. In view of this, the American Association of Study of Liver Diseases has the following guidelines for treatment of patients with HBV and HIV co-infection[54]: (1) Patients who meet criteria for chronic hepatitis B should be treated, and liver biopsy should be considered in those with fluctuating or mildly elevations in liver enzymes; (2) patients in whom treatment for both HBV and HIV is planned should receive therapies that are effective against both viruses-Lamivudine plus Tenofovir or Emtricitabine plus Tenofovir are preferred; (3) in co-infected patients with Lamivudine cross-resistance, Tenofovir or Adefovir should be added; and (4) when HAART regimens are altered, drug(s) that are effective against HBV should not be discontinued without substituting another drug that has activity against HBV, unless the patient has achieved anti-HBeAg seroconversion and has completed the prescribed course of treatment.
Furthermore, patients with HIV co-infection are less likely to develop protective HBsAb after HBV vaccination. Nevertheless, all HIV co-infected patients with serum HBsAb negativity and HBsAg positivity should be vaccinated for HBV[51,52]. Although low CD4 cell counts are associated with an impaired response to vaccination, HBV vaccination should not be deferred for patients with advanced HIV, as some individuals do develop protective antibody titers despite low CD4 cell counts. If feasible, it is recommended to vaccinate individuals with CD4 cell counts > 200/μL because response to vaccine is poor below this level. On the other hand, those with less CD4 counts should receive HAART first and HBV vaccine later when CD4 counts rise above 200/μL[55]. The patient’s HBsAb titre should be checked 1-2 mo post-vaccination, and re-vaccination should be considered for those who have not attained protective titers (≥ 10 IU/L)[47,56].
CONCLUSION
Co-infection of HBV and HIV, and the consequences on progression of severe liver diseases is a global public health issue. Despite the availability of extensive clinical data on the subject, the mechanisms of progressive hepatopathogenesis still remain inconclusive. The complexity of clinical manifestations in HBV and HIV co-infection cases therefore, offers challenging fronts to understand the basic pathobiology, and to formulate effective therapeutic strategies.
ACKNOWLEDGMENTS
The author is very grateful to Dr. Jameel S (Welcome Trust-India Alliance, India) for his continued mentoring and support. Also, the author is thankful to Dr. Gallo R (Institute of Human Virology, Baltimore, United States) for giving opportunity to work on HIV pathobiology.
P- Reviewer: Kim YJ, Meng SD S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ